I. MICROBIOTE ET GÉNÉRALITÉS
Qu’est-ce que le microbiote ?
Le microbiote définit une communauté de micro-organismes colonisant le corps humain. À tous moments de la vie, les humains sont associés à cette population des micro-organismes et à leurs produits1. Les humains ont co-évolué avec les microbes dans l'environnement, et chaque habitat corporel a un ensemble unique de micro-organismes dans son microbiote. La totalité des microbiotes présents dans le corps humain est composée de plus de 38 000 milliards de cellules, soit 0.3% du poids total du corps2. Les plus grandes concentrations de microbes occupent l'intestin, la peau et la cavité buccale et parmi les différents microbiotes, celui du tube digestif renferme le plus de micro-organismes en termes de nombre et d’espèces 2–5. En effet, le microbiote intestinal est composé de plus de mille espèces de micro-organismes, telles que des virus, des bactéries ou des champignons 6–9.
Tout d’abord identifiés comme des vecteurs de maladie au cours du XXème siècle 1011, il est désormais établi que les micro-organismes sont non seulement commensaux, c’est-à-dire qu’ils colonisent l’organisme sans causer de tort, mais surtout symbiotiques, c’est-à-dire qu’ils sont bénéfiques au bon fonctionnement du corps12.
Que fait le microbiote ?
Au niveau de l’intestin, le microbiote est impliqué dans un grand nombre de fonctions indispensables au bon fonctionnement du corps. Il intervient ainsi dans des grandes fonctions essentielles comme la nutrition, le développement, l’immunité et le bien-être 13–15. Le microbiote intestinal est donc indispensable à la dégradation des aliments, à la synthèse de vitamines et biomolécules utiles à l’organisme, à l’absorption des acides gras, du calcium et du magnésium 9,16.


Comment évolue le microbiote ?
L'exposition aux micro-organismes dès notre naissance et l'assemblage approprié du microbiote pendant l'enfance sont des processus essentiels pour établir un système immunitaire actif, nécessaire pour prévenir les maladies. Chaque individu possède une population distincte et unique de micro-organismes dans son environnement 17. Par ailleurs ce microbiote n'est pas non plus constant au cours de la vie, mais change significativement avec l'âge et en réponse aux changements de régime alimentaire. L'intestin est constamment exposé à différents éléments, qu’il s’agisse du régime alimentaire, des médicaments ingérés comme par exemple les antibiotiques ou d’agents pathogènes vecteurs de maladie. Une modification de notre mode de vie lui-même peut entrainer des modifications du microbiote intestinal, comme cela a été établi dans le cas de l'activité physique 18.
L’influence du mode de vie sur le microbiote intestinal est réellement importante. La comparaison du microbiote de populations des pays industrialisés avec celui de populations contemporaines vivant toujours de manière traditionnelle (par exemple, les chasseurs-cueilleurs) met en évidence un rôle fonctionnel différent du microbiote entre ces deux types de population. Par exemple, le microbiote ne dégradera plus les mêmes éléments ingérés, la modification fonctionnelle de l’activité du microbiote semblant liée à une perte de diversité des espèces bactériennes 19,20.
Que se passe-t-il quand le microbiote est modifié ?
La composition du microbiote change naturellement au cours du temps, cependant les modifications subies peuvent avoir des répercussions sur la santé. Il est ainsi désormais reconnu que des modifications du microbiote peuvent être impliquées dans le développement d’un grand nombre de maladies non-infectieuses21,22.
L'équilibre relatif entre les différents groupes microbiens est donc d’une importance capitale pour la santé de l'hôte. On parle de dysbiose lorsque cet équilibre est altéré et que cela abouti à une modification du fonctionnement normal de l’organisme 23. Les dysbioses peuvent être associées à diverses maladies, telles que les maladies inflammatoires de l'intestin, le diabète de type 1, la polyarthrite rhumatoïde, l'asthme ou encore l'obésité 24. Il a également été montré que le microbiote intestinal pouvait être impliqué dans le développement de cancers 25–27 et même influer sur la santé mentale en perturbant le dialogue intestin-cerveau 28–30 (Figure 1).
Comment prendre soin de son microbiote ?
Les probiotiques sont des micro-organismes vivants qui une fois administrés en quantités adéquates, confèrent des avantages pour la santé de l'hôte 32. Actuellement, les probiotiques reconnus sont représentés par les espèces microbiennes des lactobacilles, bifidobactéries, streptocoques, saccharomyces, bacilles et les entérocoques. Comme exposé précédemment, ils sont généralement apportés par les produits alimentaires fermentés, les produits alimentaires non fermentés ou les compléments alimentaires 33.
Les probiotiques agissent sur le microbiote intestinal de l'hôte par différentes modalités :
- ils améliorent la fonction de barrière protectrice jouée par l’intestin en régulant l’inflammation;
- ils améliorent la sélectivité de la barrière protectrice jouée par l’intestin en régulant l’immunité;
- ils augmentent la production de vitamines, de minéraux, d'acides gras à chaîne courte (AGCC) et de régulateurs de croissance renforçant ainsi la barrière protectrice jouée par l’intestin;
- ils augmentent l’acidité au niveau de l’intestin, favorisant ainsi son activité ;
- ils modifient la composition du microbiote intestinal.
Les modifications induites par les probiotiques permettent de prévenir et/ou de traiter des maladies aussi variées que les maladies gastro-intestinales, les hyperlipidémies et hypercholestérolémies, le cancer, l'intolérance au lactose, les maladies auto-immunes, les troubles osseux, neurodégénératifs et métaboliques33.
Il est possible de contrôler en partie l’apport en probiotiques en consommant certains fromages, yaourts ou autres produits laitiers fermentés contenant des probiotiques 34,35. Ces sources naturelles de probiotiques ont néanmoins des limitations importantes, car la composition hétérogène d'un microbiote intestinal sain nécessite une quantité et une diversité adéquates de probiotiques. Notre style de vie moderne s’accompagne souvent par des rythmes d’activité très intenses et avec peu de temps à consacrer à la sélection et à la préparation d’un régime alimentaire varié et approprié, ce qui limite le maintien d’une flore bactérienne diversifiée 36. Une autre limite de l’apport de probiotiques par l’alimentation réside dans la quantité de probiotiques naturellement présents dans les aliments, qui est très variable et souvent très faible. Dans cette perspective, les compléments alimentaires deviennent de plus en plus importants car ils fournissent une source fiable, contrôlée et conséquente de probiotiques essentiels.
II. Production et fraîcheur des probiotiques DIJO
Haute concentration de probiotiques contenus dans la formulation DIJO
La viabilité des probiotiques est essentielle pour obtenir les bienfaits associés à leur consommation. Selon les indications de la FAO / OMS, les produits probiotiques doivent contenir au moins 107 unités formant colonie (UFC) de micro-organismes vitaux par gramme à la date de péremption pour être considérés efficaces 37. Cependant la plupart des études cliniques qui ont validé les bénéfices des probiotiques chez l'homme ont utilisé des doses de probiotiques très élevées, allant de 108 à 1011 UFC 38. En fonction de la quantité ingérée et en tenant compte de l'effet du stockage sur la viabilité des probiotiques, un apport quotidien de 108-109 microorganismes probiotiques est essentiel pour obtenir une action bénéfique dans l'organisme humain, tout en maintenant un équilibre optimal entre efficacité et sécurité 39,40. En regard de ces paramètres établis par la communauté scientifique, la formulation proposée par DIJO présente une très forte concentration de probiotiques, fournissant 149 bactéries vivantes par gélule. De plus, la posologie recommandée par DIJO (2 gélules par jour) fournit un total de 289 microorganismes par jour, ce qui le place parmi les produits probiotiques les plus riches du marché (Table 1).
Avantages de la lyophilisation des probiotiques à très basse température
Pour atteindre une haute concentration finale de probiotiques, une étape cruciale est l'isolement final des bactéries de leur milieu de culture. La congélation directe des probiotiques concentrés peut être considérée comme suffisante lorsque les probiotiques sont destinés à être ajoutés dans des produits alimentaires. Cependant, si les probiotiques doivent être inclus dans une préparation pharmacologique sèche (gélules, comprimés, sachets) et éventuellement mélangés avec d'autres souches/espèces de probiotiques, les bactéries concentrées doivent être séchées. La méthode de séchage la plus économique est le séchage par aérosol, largement diffusée dans l'industrie grâce à sa rapidité et ses procédures peu coûteuses. Après concentration, les bactéries sont pulvérisées sous forme d'aérosol dans une chambre chaude qui sèche l'eau restante. Malgré ses avantages économiques, cette technique peut produire un stress nocif pour les bactéries, ce qui réduit considérablement leur disponibilité. En particulier, le stress thermique causé par une température élevée et la déshydratation sont les deux principaux mécanismes menant à l'inactivation et à la perte de viabilité des probiotiques 41.
Au lieu de cela, DIJO a opté pour la technique de la lyophilisation à très basse température, connue sous le nom de « freeze drying », qui est une procédure plus efficace pour concentrer les probiotiques, bien que plus coûteuse (Figure 2)42. Les bactéries sont d'abord congelées en-dessous d'une température critique puis séchées par sublimation sous vide en deux phases : séchage primaire, au cours duquel l'eau non liée est éliminée et séchage secondaire, au cours duquel l'eau liée est éliminée. La principale limitation de cette procédure est due à la cristallisation de l'eau et des solutés pendant le processus de congélation : la structure solide des cristaux peut endommager la membrane bactérienne et réduire la viabilité des probiotiques. Cet inconvénient est surmonté lors de la première étape de congélation par l'utilisation de cryo-préservants qui améliorent considérablement la résistance des bactéries et réduisent la cristallisation 42. Après séchage, le produit peut être broyé à une taille de particules souhaitée, qui permet de quantifier la quantité de bactéries dans la formulation finale (gélules, comprimés ou sachets) (Figure 2)43.
Plusieurs méthodes ont été développées pour délivrer les probiotiques au tractus gastro-intestinal, tels que des formulations pharmaceutiques et produits alimentaires (Table 2).
Les aliments enrichis en probiotiques disponibles dans le commerce offrent un système préférentiel pour atteindre les clients, mais il présente plusieurs limites. Tout d'abord, l'enrichissement alimentaire nécessite de nombreuses étapes pour introduire les probiotiques dans les aliments. Afin de garantir la qualité et l'efficacité thérapeutique des aliments probiotiques, il est impératif que les probiotiques conservent leur viabilité et leur intégrité tout au long du processus de fermentation alimentaire, stockage et consommation, alors qu'en fait, de multiples facteurs environnementaux peuvent miner la survie et la fonctionnalité microbiennes 45. Les variables environnementales spécifiques à chaque vecteur alimentaire différent (par exemple les jus de fruits plutôt que les produits laitiers), peuvent considérablement influencer la survie et l'efficacité des bactéries qui sont souvent liées à la courte date d'expiration des aliments. Toutes ces variables empêchent une évaluation claire des effets probiotiques, encore plus en cas de combinaison de probiotiques différents.
Dans cette perspective, les compléments alimentaires sont considérés comme plus efficaces par rapport aux systèmes de bases d’aliments enrichis 46 : les préparations probiotiques lyophilisées (capsules, comprimés ou liquides) présentent les avantages de fournir une quantité concentrée de probiotiques qui peuvent être maintenues à l'état de repos dans un environnement contrôlé. Ce type de préparation a également un meilleur succès en combinant différentes espèces/souches, car les facteurs environnementaux et le stress sont réduits au minimum 47.

Fraicheur et stabilité
Les préparations de probiotiques pures, sans ajout de conservateurs, ont l'avantage d'éviter éventuels effets collatéraux tels que l'intolérance ou la réaction allergique.
La limite de ces formulations est que les bactéries lyophilisées sont très sensibles au stockage et présentent une diminution rapide de la viabilité des micro-organismes avec le temps. Une réduction globale de 10 à 15% des cellules vivantes est généralement reconnue pour toutes les souches bactériennes lyophilisées à l'aide de la technologie de lyophilisation, mais différentes études ont identifié certaines différences spécifiques aux souches. Par exemple, une préparation de Lactobacillusacidophilus sans aucun agent de conservation a montré unediminution drastique de la viabilité des bactéries sur quatre mois d'observation à n'importe quelle température de stockage (température ambiante, réfrigérée, congelée, Figure 3)48. La sensibilité accrue aux conditions de stockage se traduit également par une diminution de l'efficacité des bactéries à survivre au niveau du transit gastro-intestinal. Une autre étude récemment menée sur des souches de lactobacilles lyophilisées (L. Plantarum et L. Rhamnosus) a évalué la survie de ces souches entre 2 et 6 mois (Figure 4). Pour cela leur efficacité/ viabilité a été mesurée après exposition à de la salive simulée (simulated saliva - SS), au liquide gastrique simulé (simulated gastric fluid - SGF), et liquide intestinal simulé (simulated intestinal fluid-SIF). Ces différents milieux ont été utilisés pour simuler le transit oro-gastro-intestinal. Les probiotiques analysés ont montré pour les deux souches une réduction importante et constante de leur capacité à survivre dans l'environnement gastro-intestinal. Les souches de Lactobacillus stockées pendant 2 mois ont conservé une viabilité ≥ 88, 66 et 45% après une exposition consécutive à SS, SS --> SGF et S-->SGF --> SIF. Les cellules lyophilisées après 4 mois de stockage présentaient une viabilité ≥ 70, 45 et 30% pendant le transit SS, SS --> SGF et SS --> SGF --> SIF respectivement. La survie des cellules lyophilisées après 6 mois de stockage était ≥ 60, 44 et 28% dans SS, SS --> SGF et SS --> SGF --> SIF respectivement 49.
Selon ces résultats, une préparation pure de probiotiques, comme celle proposée par DIJO, doit être consommée dans un délai très court après production. En effet, la production locale et la réduction des étapes dans la chaine de distribution est essentielle pour ce type de produit.
La composition du microbiote change naturellement au cours du temps, cependant les modifications subies peuvent avoir des répercussions sur la santé. Il est ainsi désormais reconnu que des modifications du microbiote peuvent être impliquées dans le développement d’un grand nombre de maladies non-infectieuses21,22.
L'équilibre relatif entre les différents groupes microbiens est donc d’une importance capitale pour la santé de l'hôte. On parle de dysbiose lorsque cet équilibre est altéré et que cela abouti à une modification du fonctionnement normal de l’organisme 23. Les dysbioses peuvent être associées à diverses maladies, telles que les maladies inflammatoires de l'intestin, le diabète de type 1, la polyarthrite rhumatoïde, l'asthme ou encore l'obésité 24. Il a également été montré que le microbiote intestinal pouvait être impliqué dans le développement de cancers 25–27 et même influer sur la santé mentale en perturbant le dialogue intestin-cerveau 28–30 (Figure 1).

III. COMBINAISON DES SOUCHES DE BACTÉRIES
Les probiotiques
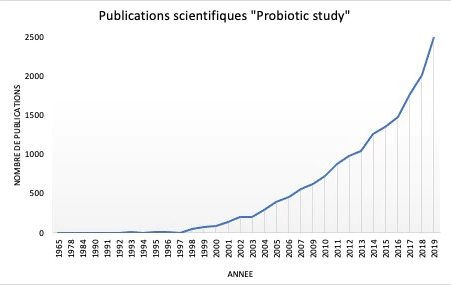
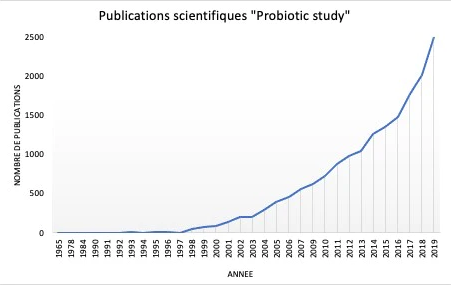
Les premières observations de bactéries bénéfiques ont été réalisées par Elie Metchnikoff en 1905, proposant pour la première fois dans l'histoire, la théorie selon laquelle les microorganismes présents dans le yogourt fermenté pouvaient être directement liés à l'augmentation de l'espérance de vie de la population bulgare 50. L'intérêt pour les aliments enrichis en probiotiques s'est accru au fil du temps, lorsque de plus en plus de publications scientifiques ont commencé à soutenir leur effet bénéfique sur la santé humaine (Figure 5) 51. Il est devenu évident que la sélection de probiotiques reposait sur des caractéristiques biologiques (sécurité alimentaire et aspects fonctionnels) et techniques. Les aspects cruciaux pour la sélection et la production de probiotiques ont rapidement été identifiés et comprenaient : la pathogénicité éventuelle, la résistance aux antibiotiques, la viabilité et la stabilité pendant le transit dans le tractus gastro-intestinal, les avantages en termes de modulation du système immunitaire, les éventuelles caractéristiques antagonistes
et la stabilité génétique et physiologique 52. En outre, la capacité d'adhésion à la muqueuse intestinale est l'un des critères de sélection les plus importants pour les probiotiques, car l'adhésion est considérée comme une nécessité pour la colonisation et est une caractéristique commune et fondamentale de tous les probiotiques.
La définition mondiale des probiotiques a finalement été établie lors d'une série de consultations d'experts conjointes de la FAO et de l'OMS (2001, 2002 et 2006) et elle précise que les probiotiques sont des "micro-organismes vivants qui, lorsqu'ils sont administrés en quantités adéquates, confèrent un avantage pour la santé de l'hôte"32 . De nombreux travaux scientifiques sur les probiotiques avaient déjà été menés à la fin des années 90 sur des modèles animaux et donné des résultats encourageants 53–55. Cependant les essais cliniques avec les probiotiques ne s'est développé que dans les premières décennies des années 200 et a connu une croissance exponentielle, après que l'OMS ait reconnu les effets potentiels du traitement aux probiotiques sur leur capacité à :
- rétablir le nombre de bactéries à la base naturellement présentes dans une niche, nombre qui a pu diminuer et causer une maladie
- contrecarrer des pathogènes et une/des maladies, permettant le rétablissement du patient et de sa flore microbienne
Aujourd'hui, les effets des probiotiques sur le microbiote intestinal et la santé en général sont bien connus. Les probiotiques présentent un large éventail d'effets bénéfiques à la fois locaux, sur le biome intestinal, et systémiques, sur l'immunosystème et le métabolisme (Figure 5). En raison de l'hétérogénéité des organismes utilisés dans les traitements probiotiques, ces effets peuvent varier d'une souche ou d'une espèce à l'autre ou être plus spécifiques pour certaines souches ou espèces : la combinaison de différentes sources de probiotiques devient de plus en plus la meilleure approche pour obtenir des résultats synergiques 56.
Quels avantages à combiner différents probiotiques ?
Depuis le début des années 90, la communauté scientifique est de plus en plus consciente que les
combinaisons de différentes souches et espèces de probiotiques sont plus efficaces que l'administration d'un
seul. Au cours de cette période, le groupe de Pesce de Ruiz Holgado a mené une série d'expériences sur
différents modèles in vivo, au cours desquelles lui et son collaborateur ont évalué l'effet d'un traitement
probiotique simple et multi-souches sur les infections bactériennes 55. En particulier, ils ont pu montrer que
Lactobacillus acidophilus et Lactobacillus casei pouvaient mieux protéger les souris contre l'infection à Salmonella Typhimurium lorsqu'ils sont combinés, plutôt qu'individuellement. Les souris ont reçu pendant 8 jours l’un des produits fermentés (seul ou en combinaison, 20 % de suspension dans de l'eau potable, ce qui donne un total de 2,4×109 organismes viables), suivi d'une infection orale avec S. Typhimurium. Les souris nourries avec la combinaison des deux probiotiques ont montré un taux de survie de 100% 21 jours après l'infection, comparé aux contrôles (nourris avec du lait écrémé) ou au traitement probiotique unique (20% de taux de survie). Le taux de survie a été caractérisé par une meilleure réponse immunitaire face à l'infection et une meilleure élimination des particules de salmonelles du foie. Ces résultats ont été rapidement confirmés par la publication de (Paubert-Braquet et al, 1995) 54 qui ont observé une résistance accrue au S. Typhimurium chez des souris nourries avec des laits fermentés contenant un mélange de différentes espèces de
Lactobacillus (1,1×108 de St. thermophilus, 8,2×108 de Lb. bulgaricus, 0,8×108 de Lb. casei). La survie observée était de 87,5 % après 14 jours par rapport au 0 % du groupe contrôle, le taux de survie avec un traitement
Lactobacillus casei seul étant significativement plus faible.
Aujourd'hui, il est devenu évident que les suppléments multi-espèces ayant des caractéristiques
différentes ont une meilleure chance de colonisation et présentent des effets synergiques et une meilleure
chance de survie et d'adhésion. En outre, les relations positives entre les souches peuvent augmenter leur
activité biologique 57. De nombreuses études ont été menées l'année dernière pour évaluer l'effet possible des combinaisons de probiotiques non seulement dans le traitement des symptômes pathologiques, mais
aussi dans la perspective de préserver un mode de vie sain. Les études sur la modulation probiotique des paramètres physiologiques (poids corporel, pression artérielle, glycémie), les performances sportives et les
processus cognitifs sont particulièrement intéressantes.
i) Combinaison de probiotiques dans le contrôle des paramètres physiologiques
(poids corporel, pression sanguine et glycémie)
Les changements d'habitudes alimentaires et la disponibilité accrue d'aliments à haute teneur en
calories ont fait du surpoids et de l'obésité l'un des problèmes de santé les plus graves de notre époque.
L'Organisation mondiale de la santé (OMS) a estimé que 39 % des personnes de plus de 18 ans étaient en
surpoids, et la prévalence mondiale de l'obésité a presque triplé depuis 1975. Près de 2,8 millions de décès
par an sont la conséquence de la surcharge pondérale et des affections associées à l'obésité, telles que la
dyslipidémie, l'hypertension artérielle et l'insulino-résistance, qui entraînent un risque de maladie
coronarienne, d'accident vasculaire cérébral ischémique, de diabète sucré de type 2 et de cancer (statistiques
de l'OMS, 2020). Toutes ces caractéristiques peuvent être liées à un déséquilibre des microbiotes intestinaux
qui sont la conséquence de l'obésité et de la surcharge pondérale 58.
De nombreuses études ont été menées ces dernières années sur de grandes cohortes, mais les
principales études de méta-analyse croisant tous les résultats obtenus s'accordent à dire que les mélanges
probiotiques multi-souches et multi-espèces sont plus performants que les souches uniques dans le contrôle
du poids corporel. (Zhang et al., 2016) ont analysé un total de 25 études impliquant 1931 personnes et ont
montré que la consommation de mélanges de probiotiques pouvait réduire de manière significative l'indice
de masse corporelle (-0,65 de l'IMC avec une valeur p <0,01) par rapport à une réduction moins importante
et non statistiquement significative pour les souches uniques (-0,12 IMC avec une valeur p = 0,17) (voir Table
3) 59. Ces effets sur les résultats étaient plus évidents dans la population obèse et pendant une longue période
d'administration (>8 semaines) (voir Table 3). Une conclusion similaire a été tirée par la méta-analyse de
(Koutnikova et al., 2019) sur des cohortes plus importantes de publications (105) impliquant plus de 6800
patients : les améliorations de l'IMC et de la glycémie ont été principalement observées avec la combinaison
de plusieurs souches de bifidobactéries (Bifidobacterium breve, B. longum), St. thermophilus et L. acidophilus,
L. casei, L. delbrueckii.
60
La modulation du poids corporel est souvent associée à l'amélioration d'autres paramètres
systémiques tels que la pression artérielle et la glycémie. Une méta-analyse récente portant sur 9 études
différentes des effets des probiotiques sur la pression artérielle a révélé que la consommation de
probiotiques réduisait sensiblement la pression artérielle systolique globale de 3,56 mmHg et la pression
artérielle diastolique de 2,38 mmHg par rapport aux groupes de contrôle. Cette méta-analyse suggère que
les meilleurs résultats ont été obtenus dans la réduction des conditions préexistantes d'hypertension
(patients hypertendus), lorsque plusieurs espèces de probiotiques étaient consommées et que la durée de
l'intervention était de ≥8 semaines61. Ces mêmes paramètres de composition du mélange de probiotiques
(probiotiques multi-souches contenant L. acidophilus, S. thermophilus, L. bulgaricus, et/ou B. lactis) et de
durée d'administration ont également permis de diminuer la concentration de glycémie chez les patients
atteints de diabète de type II, puisque les espèces Lactobacillus, Bifidobacterium, et Streptococcus sont
connues pour réguler le contrôle glycémique via la signalisation de la satiété, l'intégrité intestinale, et la
protection antioxydante des cellules pancréatiques 62.
ii) Avantage de la combinaison de probiotiques dans la pratique du sport
Les symptômes gastro-intestinaux (GI) et des voies respiratoires supérieures (VRS) sont des malaises
courants chez les athlètes d'endurance. Plusieurs recherches ont montré des résultats différents des
probiotiques sur ces symptômes, probablement liés à la souche probiotique, à la dose, à la période de
consommation ou même à la forme d'administration (capsules, sachets ou lait fermenté). Cependant il existe
un accord commun sur le fait que les souchesingérées en combinaison sur une période de consommation
plus longue peuvent montrer de meilleurs résultats pour minimiser les symptômes VRS et GI 63.
Des suppléments probiotiques multisouches contenant les espèces L. acidophilus, B. bifidum et B.
animalis ssp lactis ont été testés dans de nombreux essais, traitements d’au moins un mois. Ces études ont
fait état de résultats positifs, caractérisés par une meilleure préservation de la barrière intestinale en cas
d'efforts prolongés (Lamprecht et Frauwallner, 2012) et des concentrations plasmatiques d'endotoxines plus
faibles 64,65. (Strasser et al., 2016), a rapporté que des probiotiques multi-souches étaient capables de réduire
l'occurrence de symptômes dans les VRS de 2,2 fois après 12 semaines dans le groupe traité par rapport au
placebo. Cette amélioration a été corrélée à une réduction significative du tryptophane, de la phénylalanine
et de leurs catabolites primaires dans le sang66.
iii) Effet des probiotiques sur les processus cognitifs et l'humeur
Il a été démontré que le microbiote intestinal influençait activement le bon développement et le bon
fonctionnement du système immunitaire et du cerveau dans ce que l'on appelle aujourd'hui l'axe "microbiote-
intestin-cerveau" (MIC). Un changement défavorable du microbiote intestinal perturbe la symbiose
maintenue par l'axe MIC et a été associé à des maladies graves comme la schizophrénie, la maladie de
Parkinson et la dépression majeure 67. Les patients souffrant de ce trouble présentaient souvent des niveaux
réduits des deux principaux genres de probiotiques, Lactobacillus et Bifidobacterium 68. Même si un
traitement avec des souches simples permet de mieux comprendre la fonction et la contribution de chaque
probiotique, les probiotiques multi-souches peuvent être plus puissants chez l'homme. En effet, différentes
combinaisons de L. acidophilus, B. bifidum, St. thermophile, L. casei et B. bifidum ont montré à plusieurs
reprises des effets antidépresseurs positifs 69,70. Des résultats positifs ont également été obtenus sur de
jeunes étudiants (en moyenne 20 ans) qui ont montré une amélioration de la panique et de l'anxiété
neurophysiologique, l’affect négatif, de l'inquiétude et une augmentation de la régulation négative de
l'humeur après avoir reçu régulièrement des probiotiques multi-souches 71. Les mêmes résultats bénéfiques
ont été obtenus par (Akkasheh et al., 2016) où un traitement de 12 semaines avec L. acidophilus, L. casei, B.
bifidum, et Lactobacillus fermentum a induit une amélioration sensible des fonctions cognitives et du statut
métabolique de patients âgés atteints d’Alzheimer69.
Caractéristiques des différentes souches probiotiques utilisés par DIJO
Les sources de probiotiques les plus populaires sélectionnées pour leurs propriétés biologiques sont
constituées par les genres Lactobacillus, Bifidobacterium, Streptococcus, Saccharomyces, Bacillus,
Enterococcus. Ces espèces sont largement utilisées dans les produits alimentaires fermentés, les produits
alimentaires non fermentés, ainsi que dans les compléments alimentaires fonctionnels et nutraceutiques 72.
Les bifidobactéries et les lactobacilles sont les plus représentées dans le tractus intestinal des
humains et des animaux et, par conséquent, les plus utilisées dans la production d'aliments enrichis. Ces
deux espèces peuvent facilement être confondues, mais elles présentent des différences métaboliques qui
les rendent complémentaires en termes de survie et de fonctions. Par exemple, par rapport aux lactobacilles,
les bifidobactéries ne sont pas aussi tolérantes aux acides et leur croissance ne peut être qualifiée d’
« anaérobie facultative ». En effet, les bifidobactéries produisent de l'acide lactique comme les lactobacilles à
partir de la fermentation des hydrates de carbone, mais en plus, elles produisent de l'acide acétique qui
nécessite des voies cataboliques spécifiques non partagées avec les bactéries lactiques.
a. Bifidobacterium
nouveau-nés. Les bifidobactéries sont des cellules gram-positives, courbées et bifurquées (fendues, en forme
de X ou de Y) en forme de bâtonnet. On les trouve principalement dans l'environnement intestinal humain et
animal : l'intestin humain, l'intestin animal (murin, lapin, bovin, poulet et insecte) et la cavité buccale. Les
bifidobactéries sont largement répandues parmi les organismes vivants qui fournissent des soins parentaux
à leur progéniture, tels que les mammifères, les oiseaux et les insectes sociaux, mais n'ont jamais été trouvées
chez d'autres animaux comme les poissons et les reptiles. Compte-tenu de cette présence exclusive, la
transmission directe de cellules bifidobactériennes des parents/soignants à la progéniture pourrait être une
raison importante de leur répartition écologique 73.
Le genre comprend 32 espèces décrites. Dans la fabrication de produits laitiers fermentés dérivés du
lait, Bifidobacterium bifidum est l'espèce la plus utilisée, tandis que Bifidobacterium longum et
Bifidobacterium infantis sont les espèces prédominantes dans les selles des nourrissons allaités 73. Le B.
bifidum et le B. infantis sont appréciés pour leur capacité à synthétiser des quantités appréciables de
certaines vitamines, telles que la thiamine, les acides foliques, la biotine et les acides nicotiniques 74.
i) Bifidobacterium bifidum
Bifidobacterium bifidum est une souche probiotique qui est utilisée comme ingrédient majeur pour la fabrication de produits nutraceutiques et comme produit laitier de démarrage depuis les années 2000. Les divers effets bio-fonctionnels et le potentiel d'application industrielle de B. bifidum ont été caractérisés et prouvés par des études in vitro et in vivo et des études cliniques (Table 4). La bactérie B. bifidum est très utile pour améliorer les processus métaboliques de l'intestin (comme le métabolisme des glucides, la production de vitamines et la dégradation des catabolites d'origine alimentaire). Elle est également utile pour renforcer le système immunitaire de l'hôte en contrôlant la composition du biome de l'intestin et en interférant directement avec les cellules intestinales. Plusieurs études ont suggéré le rôle important de B. bifidum comme anticancéreux 77 et comme renforçateur du système immunitaire 78,79. Ces observations ont été confirmées par des essais cliniques où B. bifidum a montré des effets bénéfiques dans la réduction des symptômes des maladies allergiques/auto-immunes 80,81, le syndrome du côlon irritable 82 et l'atténuation de la dégénérescence cognitive dans les maladies liées au vieillissement comme la maladie d'Alzheimer 83.
ii) Bifidobacterium infantis (32624)
Bifidobacterium longum subsp infantis 35624 (B. infantis) a été isolée à l'origine à partir de tissus gastro-intestinaux humains sains réséqués il y a environ 15 ans. C’est la souche la plus représentée dans l'intestin des nourrissons. Dans la muqueuse gastro-intestinale, les cellules épithéliales sont les premières à rencontrer des microbes. Il a été démontré que B. infantis adhère aux lignées de cellules épithéliales gastro-intestinales, sans induire l'activation de NFKB ou la sécrétion de chimiokines93, suggérant que ce microbe exerce des effets immunomodulateurs sur les cellules intestinales qui servent de médiateurs aux réponses pro-inflammatoires de l'hôte aux agents pathogènes entériques 94.
Les effets les plus étudiés de B. infantis concernent sa capacité
à moduler la réponse immunitaire de l'hôte en différenciant directement
la population lymphocytique immature (cellule T ; cellules résidentes du thymocyte), via une interaction cellulaire dendritique, vers les cellules T-REG, impliquées dans le mécanisme de tolérance. En conséquence, la différenciation vers les lymphocytes immunoréactifs (TH, cellules auxiliaires T) est significativement réduite sous l'effet de la B. infantis. Cette observation a été validée par l'administration de B. infantis à des volontaires sains : en effet, le groupe traité a montré une augmentation significative des cellules T-REG dans le sang par rapport au groupe contrôle ainsi qu’une réduction cohérente des cytokines inflammatoires circulantes 95. L'application clinique de ces résultats chez des patients humains a montré un effet positif sur les pathologies liées à une activation accrue du système immunitaire et de l'inflammation. En particulier, l'administration de B. infantis a amélioré les symptômes lors
de réactions allergiques 96,97, chez les patients souffrant du syndrome du côlon irritable chez l'adulte 97, de diarrhée et d'infection intestinale chez les nouveau-nés 98,99(Figure 7).
iii) Bifidobacterium longum
Bifidobacterium longum est la bactérie la plus courante présente chez les nouveau-nés et représente, avec d'autres espèces de Bifidobacterium, jusqu'à 90% des bactéries du tractus gastro-intestinal d'un nourrisson qui tombe à 3% dans le tractus gastro-intestinal d'un adulte 101. B. longum est considéré comme une bactérie versatile car elle est capable de métaboliser plusieurs substrats. Cette capacité est liée au nombre élevé de gènes associés au métabolisme des oligosaccharides acquis à la suite de duplications de gènes et de transferts de gènes horizontaux, indiquant que B. longum est soumise à une pression sélective pour augmenter sa capacité à entrer en compétition pour divers substrats dans le tractus gastro-intestinal102.
Les capacités métaboliques des glucides de la sous-espèce longum semblent être plus orientées vers le métabolisme des glucides végétaux complexes et n'ont pas la même capacité que la sous-espèce infantis à dégrader les oligosaccharides du lait maternel humain. 103. Il a été découvert que certaines souches de B. longum avaient une tolérance élevée à l'acide gastrique et à la bile, ce qui suggère que ces souches pourraient
survivre dans le tractus gastro-intestinal pour coloniser les petits et gros intestins. En fait, le longum est également riche en hydrolases de sels biliaires permettant l'hydrolyse des sels biliaires en acides aminés et
en acides biliaires. Cette fonction n'est pas encore totalement claire, bien que B. longum puisse utiliser les produits d'acides aminés pour mieux tolérer les sels biliaires.104
Des essais cliniques récents sur B. longum ont montré des effets bénéfiques dans le traitement des patients atteints du syndrome du côlon irritable 105,106. Différentes études in vivo suggèrent un rôle possible de cette bactérie dans la modulation de la réponse auto-immune dans modèles de diabète de type II et des pathologies immunologiques dues à l’intolérance au gluten107,108.
b. Lactobacillus
Le genre Lactobacillus comprend plus de 200 espèces caractérisées par une diversité phylogénétique et métabolique qui dépasse celle d'une famille bactérienne typique. Ces bactéries sont très dépendantes des nutriments, de sorte qu'on les trouve préférentiellement dans les organes de transformation des aliments des humains, des rongeurs, des animaux de ferme, mais aussi des insectes et des oiseaux 109 (Figure 8). Les lactobacilles peuvent être subdivisés en sous-familles en fonction de leur dépendance à l'égard de l'hôte : les bactéries vivant en liberté se trouvent dans l'environnement (herbe, sol) et sont dotées d'un ensemble de gènes relativement important ; les bactéries nomades ne sont pas autonomes et colonisent différents organes de l'hôte et elles peuvent migrer dans différentes niches ; enfin, les bactéries spécifiques à l'hôte qui sont hautement adaptées pour fonctionner chez des hôtes spécifiques 110. Ces comportements métaboliques reflètent la taille de leur génome : le génome large des groupes vivant en liberté et des groupes nomades leur confère plus de flexibilité et d'adaptabilité, tandis que le génome limité des gènes spécifiques de l'hôte leur confère une efficacité métabolique supérieure, mais limitée à un environnement spécifique. Outre les caractéristiques communes au métabolisme des lactobacilles, certaines caractéristiques sont spécifiques à chaque sous-type. Dans le groupe des nomades, on trouve certaines espèces de Lactobacillus, comme L. plantarum, L. casei, L. paracasei et L. rhamnosus, qui, bien que non autochtones au sens strict, possèdent des adaptations aux écosystèmes intestinaux et à la cavité buccale, leur permettant de persister au moins pendant un temps limité.
Les bactéries probiotiques les plus couramment utilisées appartiennent aux bactéries lactiques, et plus particulièrement à celles des anciens genres Lactobacillus et Enterococcus111. La production de peptides bioactifs à partir des bactéries lactiques est souvent liée aux avantages pour la santé. Les peptides bioactifs sont libérés des protéines par hydrolyse enzymatique microbienne ou non microbienne 112. Les peptides bioactifs peuvent agir comme modulateurs immunitaires 113, et sont antihypertenseurs en inhibant l'enzyme de conversion de l'angiotensine (ECA) 114, comme dans les yaourts115, et les antioxydants comme dans le levain 116 et le yaourt aussi117. Des peptides antioxydants ont été trouvés dans le lait de soja fermenté par les bactéries lactiques et peuvent éliminer efficacement les radicaux libres118. Les propriétés antioxydantes peuvent également être la conséquence de la production de polysaccharides extracellulaires par les bactéries qui les sécrètent dans la lumière du tube digestif 119.
D'autres avantages pour la santé sont liés à la consommation de produits fermentés à l'aide de souches probiotiques bactéries lactiques. En effet, la consommation de yaourts a donné des résultats intéressants par rapport au diabète de type 2 dans une méta-analyse de la consommation de produits laitiers 120. Les bactéries lactiques produisent également des composés antimicrobiens dont les propriétés peuvent également contribuer à l'établissement de souches probiotiques chez l'hôte 121 et ainsi contrer les bactéries pathogènes dans le tractus gastro-intestinal.
i) Lactobacillus casei group (LCG) : Lactobacillus casei, paracasei et rhamnosus
Le groupe Lactobacillus casei (LCG), composé principalement des espèces étroitement apparentées L. casei, L. paracasei et L. rhamnosus, est l'une des espèces les plus étudiées en raison de son potentiel commercial, industriel et sanitaire. Sur le plan commercial, elles sont utilisées pour fermenter les produits laitiers, ce qui permet souvent d'améliorer la saveur et la texture des aliments. Les bactéries LCG présentent plusieurs avantages intrinsèques qui en font l'un des probiotiques les plus exploités : en effet, les LCG ont une caractéristique métabolique particulière qui leur confère une grande résistance à l'environnement acide de l'intestin 1, au stress oxydatif dérivé du métabolisme 123 et aux variations osmotiques et de température pendant les processus de production 124,125.
Les mécanismes par lesquels ces bactéries ont, directement ou indirectement, un effet bénéfique sur la santé humaine ne sont pas encore totalement compris et nécessitent des études plus approfondies. Les mécanismes potentiels comprennent la production de substances antimicrobiennes telles que les bactériocines, le renforcement de la barrière épithéliale par l'attachement, la compétition pour les sites de liaison pathogènes ou la modulation du système immunitaire 126.
Les bactéries LCG ont été largement étudiées pour leurs propriétés potentielles dans l'amélioration de la santé humaine dans différents domaines, tels que les allergies, le cancer de l'obésité et le dérèglement
du microbiote intestinal (Table 5) 127.
ii) Lactobacillus plantarum
Lactobacillus plantarum est un Lactobacillus très diffus, présent dans un large spectre de produits
laitiers, de légumes, de viande, d'ensilage, de vin et dans les voies gastro-intestinales, vaginales et urogénitales 140. La présence omniprésente de L. plantarum est soutenue par des capacités d'adaptation et une voie métabolique élevées 141. L. plantarum a été initialement sélectionnée pour sa capacité à maintenir l'intégrité des produits laitiers contre des micro-organismes contaminants 142. Ces fonctions antifongiques et antimicrobiennes reposent sur la production élevée d'une classe particulière de toxines bactériennes, les bactériocines, qui ont rendu le L. plantarum intéressant à la fois pour l'industrie alimentaire et les compléments probiotiques. En effet L. plantarum colonise le tube gastrique de l'hôte et offre une protection contre les pathogènes gastro-intestinaux (Table 6) 143.
L. plantarum a montré un effet important dans la réduction de l'effet de certaines pathologies humaines affectant le tractus grasto-intestinal. Des études cliniques ont montré les effets bénéfiques de L. plantarum dans la diarrhée dérivée des antibiotiques 144, le diabète et le métabolisme des graisses 145.
D'autres preuves relient l'homéostasie de la population microbienne intestinale au bien-être mental. Dans cette perspective, l'utilisation du L. plantarum sur des patients atteints de dépression majeure, en plus du traitement chimique standard, s'est révélée efficace pour réduire la concentration sanguine de kynorunine et améliorer les fonctions cognitives 146,147.
iii) Lactobacillus acidophilus
Le Lactobacillus acidophilus appartient au groupe spécifique des Lactobacilles et présente des différences importantes par rapport au groupe de L. casei et de L. Plantarum. En fait, L. acidophilus présente un génome limité, comparé aux lactobacilles nomades, qui fournit un ensemble de gènes conférant une grande efficacité de survie dans un nombre limité de niches. Comme son nom l'indique, il colonise facilement un environnement acide que l'on peut trouver, dans le corps humain, dans le tube gastrique (de la cavité
buccale au côlon) et dans l'environnement vaginal. Cette espèce de bactérie est l'une des premières à avoir fait l'objet d'études approfondies pour des applications dans les produits laitiers et l'amélioration de la santé,
présentant une large littérature déjà avant les années 2000 154. Les L. acidophilus présentent des caractéristiques communes aux autres lactobacilles, telles qu'une meilleure adhérence aux cellules épithéliales intestinales (Buck et al., 2005), une résistance extrême aux acides et aux sels biliaires 155 et la
production de bactériocine antibiotique 154.
L. acidophilus a été testée dans des essais cliniques qui montrent comment elle peut influencer l'homéostasie systémique et améliorer les symptômes de plusieurs syndromes métaboliques. La consommation de probiotiques contenant L. acidophilus en combinaison avec des espèces de Bifidobacterium réduisent les ballonnements chez les adultes souffrant de troubles fonctionnels intestinaux 156. Les propriétés antibiotiques et anti-inflammatoires de L. acidofilus ont contribué à réduire l'incidence et la durée des symptômes, l'incidence du rhume et de la grippe sur prescription d'antibiotiques chez les enfants âgés de 3 à 5 ans 157. L. acidophilus en complément avec d'autres lactobacilles s'est également révélée efficace pour réduire les symptômes des pathogènes bactériens dans les vaginoses 158 et les infections à Clostridium difficile159. Des expériences in vitro ont également suggéré un effet anticancéreux, corrélé à la production d'acide linoléique conjugué et à l'élimination possible de sous-produits métaboliques pro-cancérogènes 160.
c. Lactococcus lactis
Lactococcus est un genre de bactéries lactique et est connu sous le nom d'homofermentateur, car il produit presque exclusivement de l'acide lactique à l’issue de la fermentation du glucose. Son caractère homofermentaire peut être modifié en ajustant les conditions environnementales telles que le pH, la concentration de glucose et la limitation des nutriments. Récemment, les Lactococcus lactis sont passés d'agents utilisés dans l'industrie alimentaire à des véhicules qualifiés pour l'administration de médicaments par les muqueuses grâce à leur capacité développée à lier les surfaces des muqueuses et à modifier les réponses immunitaires (Figure 9) 161. De nombreuses souches modifiées ont été produites les dernières années dans le but de délivrer directement des protéines et de l'ADN à la muqueuse des voies respiratoires et digestives afin d'améliorer la tolérance des muqueuses. Ce processus implique la capacité de l'antigène
administré par la muqueuse à réguler les réponses immunitaires locales et systémiques162.
De nombreux tests expérimentaux ont été réalisés ces dernières années avec des résultats encourageants. Par exemple, L. lactis NCDO 2118 réduit les symptômes de la colite récurrente dans un modèle in vivo de colite induite. La production précoce d'IL-6 renforcée par la bactérie joue un rôle dans l'amélioration de la réparation des muqueuses et la préservation de la production d'IL-10 du côlon, ce qui pourrait être à l'origine d'importants effets anti-inflammatoires 163. En outre, un traitement oral avec la même
souche de L. Lactis a presque doublé les fréquences des cellules T-REG dans les ganglions lymphatiques mésentériques et la rate. De nombreuses études ont montré que L. lactis peut soutenir la fonction barrière en termes d'amélioration du mucus, de production de peptides antimicrobiens et de sécrétion d'immunoglobuline soluble 164. Plusieurs essais cliniques sont actuellement en cours pour valider le traitement de L. lactis modifiée chez l'homme.

BIBLIOGRAPHIE
1. Costello, E. K. et al. Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697 (2009).
2. Sender, R., Fuchs, S. & Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol 14, (2016).
3. Eckburg, P. B. et al. Diversity of the Human Intestinal Microbial Flora. Science 308, 1635–1638 (2005).
4. Lay, C. et al. Colonic Microbiota Signatures across Five Northern European Countries. Appl Environ Microbiol 71,
4153–4155 (2005).
5. Pei, Z. et al. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci U S A 101, 4250–4255 (2004).
6. Gerritsen, J., Smidt, H., Rijkers, G. T. & de Vos, W. M. Intestinal microbiota in human health and disease: the impact
of probiotics. Genes & Nutrition 6, 209–240 (2011).
7. Lagier, J.-C. et al. Culture of previously uncultured members of the human gut microbiota by culturomics. Nature
Microbiology 1, 16203 (2016).
8. Ley, R. E., Lozupone, C. A., Hamady, M., Knight, R. & Gordon, J. I. Worlds within worlds: evolution of the
vertebrate gut microbiota. Nat Rev Microbiol 6, 776–788 (2008).
9. Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K. & Knight, R. Diversity, stability and resilience of the
human gut microbiota. Nature 489, 220–230 (2012).
10. Pasteur, L. Remarks on Anthracic Vaccination as a Prophylactic of Splenic Fever. Br Med J 1, 489 (1882).
11. Koch, R. Die Ätiologie der Tuberkulose. in Beiträge zur Biologie der Pflanzen vol. Bd. 2, Heft 2 (Robert Koch-
Institut, 1876).
12. Xu, J. & Gordon, J. I. Honor thy symbionts. Proceedings of the National Academy of Sciences 100, 10452–10459
(2003).
13. Archie, E. A. & Theis, K. R. Animal behaviour meets microbial ecology. Animal Behaviour 82, 425–436 (2011).
14. McFall-Ngai, M. et al. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci U S
A 110, 3229–3236 (2013).
15. Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal
microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
16. Brown, J. M. Eating to boost gut microbial diversity. Science Translational Medicine 8, 369ec198 (2016).
17. Meadow, J. F. et al. Humans differ in their personal microbial cloud. PeerJ 3, e1258 (2015).
18. Petriz, B. A. et al. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats.
BMC Genomics 15, (2014).
19. Iebba, V. et al. Eubiosis and dysbiosis: the two sides of the microbiota. New Microbiol 39, 1–12 (2016).
20. Zaneveld, J. R., McMinds, R. & Vega Thurber, R. Stress and stability: applying the Anna Karenina principle to
animal microbiomes. Nat Microbiol 2, 17121 (2017).
21. Farthing, M. J. G. Bugs and the gut: an unstable marriage. Best Pract Res Clin Gastroenterol 18, 233–239 (2004).
22. Guarner, F. & Malagelada, J.-R. Gut flora in health and disease. The Lancet 361, 512–519 (2003).
23. Tamboli, C. P., Neut, C., Desreumaux, P. & Colombel, J. F. Dysbiosis in inflammatory bowel disease. Gut 53, 1–4
(2004).
24. Keeney, K. M., Yurist-Doutsch, S., Arrieta, M.-C. & Finlay, B. B. Effects of Antibiotics on Human Microbiota and
Subsequent Disease. Annu. Rev. Microbiol. 68, 217–235 (2014).
25. Ray, D. & Kidane, D. Gut Microbiota Imbalance and Base Excision Repair Dynamics in Colon Cancer. J Cancer 7,1421–1430 (2016).
26. Wroblewski, L. E., Peek, R. M. & Coburn, L. A. The Role of the Microbiome in GI Cancer. Gastroenterol Clin North
Am 45, 543–556 (2016).
27. Yamamoto, M. & Matsumoto, S. Gut microbiota and colorectal cancer. Genes Environ 38, 11 (2016).
28. Moos, W. H. et al. Microbiota and Neurological Disorders: A Gut Feeling. Biores Open Access 5, 137–145 (2016).
29. Mu, C., Yang, Y. & Zhu, W. Gut Microbiota: The Brain Peacekeeper. Front Microbiol 7, 345 (2016).
30. Obata, Y. & Pachnis, V. The Effect of Microbiota and the Immune System on the Development and Organization of
the Enteric Nervous System. Gastroenterology 151, 836–844 (2016).
31. Thomas, S. et al. The Host Microbiome Regulates and Maintains Human Health: A Primer and Perspective for Non-
Microbiologists. Cancer Res 77, 1783–1812 (2017).
32. Joint FAO/WHO Expert Consultation. Probiotics in food : health and nutritional properties and guidelines for
evaluation : Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics
in Food including Powder Milk with Live Lactic Acid Bacteria, Cordoba, Argentina, 1-4 October 2001 [and] Report of a Joint
FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food, London, Ontario, Canada, 30
April -1 May 2002. (2006).
33. M, A., N, M. & M, S. Therapeutic, Prophylactic, and Functional Use of Probiotics: A Current Perspective. Frontiers
in microbiology vol. 11 https://pubmed.ncbi.nlm.nih.gov/33042069/ (2020).
34. Bonifait, L., Chandad, F. & Grenier, D. Probiotics for oral health: myth or reality? J Can Dent Assoc 75, 585–590
(2009).
35. Parvez, S., Malik, K. A., Ah Kang, S. & Kim, H.-Y. Probiotics and their fermented food products are beneficial for
health. J Appl Microbiol 100, 1171–1185 (2006).
36. Bagchi, T. Traditional food & modern lifestyle: Impact of probiotics. The Indian Journal of Medical Research 140,
333 (2014).
37. Morelli, L. & Capurso, L. FAO/WHO Guidelines on Probiotics: 10 Years Later. Journal of Clinical Gastroenterology
46, S1 (2012).
38. Ouwehand, A. C. A review of dose-responses of probiotics in human studies. Benef Microbes 8, 143–151 (2017).
39. Hill, C. et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope
and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology 11, 506–514 (2014).
40. Knorr, D. Technology aspects related to microorganisms in functional foods. Trends in Food Science & Technology
9, 295–306 (1998).
41. Santivarangkna, C., Kulozik, U. & Foerst, P. Inactivation mechanisms of lactic acid starter cultures preserved by
drying processes. Journal of Applied Microbiology 105, 1–13 (2008).
42. Broeckx, G., Vandenheuvel, D., Claes, I. J. J., Lebeer, S. & Kiekens, F. Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. International Journal of Pharmaceutics 505, 303–318 (2016).
43. Forssten, S. D., Laitila, A., Maukonen, J. & Ouwehand, A. C. Probiotic triangle of success; strain production, clinical
studies and product development. FEMS Microbiol Lett 367, (2020).
44. Broeckx, G., Vandenheuvel, D., Claes, I. J. J., Lebeer, S. & Kiekens, F. Drying techniques of probiotic bacteria as
an important step towards the development of novel pharmabiotics. International Journal of Pharmaceutics 505, 303–318
(2016).
45. Fiocco, D. et al. Characterization of the CtsR Stress Response Regulon in Lactobacillus plantarum. Journal of
Bacteriology 192, 896–900 (2010).
46. Khedkar, S., Carraresi, L. & Bröring, S. Food or pharmaceuticals? Consumers’ perception of health-related borderline
products. PharmaNutrition 5, 133–140 (2017).
47. Putta, S. et al. Probiotics: Supplements, Food, Pharmaceutical Industry. in Therapeutic, Probiotic, and
Unconventional Foods 15–25 (Elsevier, 2018). doi:10.1016/B978-0-12-814625-5.00002-9.
48. Menezes, M. F. da S. C. de et al. Improvement of the viability of probiotics (Lactobacillus acidophilus) by multilayer
encapsulation. Ciência Rural 49, (2019).
49. Shekh, S. L., Boricha, A. A., Chavda, J. G. & Vyas, B. R. M. Probiotic potential of lyophilized Lactobacillus
plantarum GP. Annals of Microbiology 70, 16 (2020).
50. Metchnikoff, E. & Mitchell, P. C. (Peter C. The prolongation of life; optimistic studies. (New York & London : G.P.
Putnam’s Sons, 1908).
51. Siró, I., Kápolna, E., Kápolna, B. & Lugasi, A. Functional food. Product development, marketing and consumer
acceptance--a review. Appetite 51, 456–467 (2008).
52. Saarela, M., Mogensen, G., Fondén, R., Mättö, J. & Mattila-Sandholm, T. Probiotic bacteria: safety, functional and
technological properties. J Biotechnol 84, 197–215 (2000).
53. McFarland, L. V. & Elmer, G. W. Pharmaceutical probiotics for the treatment of anaerobic and other infections.
Anaerobe 3, 73–78 (1997).
54. Paubert-Braquet, M., Xiao-Hu, G., Gaudichon, C., Hedef, N. & Serikoff, A. Enhancement of host resistance against
Salmonella typhimurium in mice fed a diet supplemented with yogurt or milks fermented with various Lactobacillus casei
strains. International journal of immunotherapy 11, 153–161 (1995).
55. Perdigon, G., Nader de Macias, M. E., Alvarez, S., Oliver, G. & Pesce de Ruiz Holgado, A. A. Prevention of
gastrointestinal infection using immunobiological methods with milk fermented with Lactobacillus casei and Lactobacillus
acidophilus. J Dairy Res 57, 255–264 (1990).
56. Nagpal, R. et al. Probiotics, their health benefits and applications for developing healthier foods: a review. FEMS
Microbiol Lett 334, 1–15 (2012).
57. Chapman, C. M. C., Gibson, G. R. & Rowland, I. Health benefits of probiotics: are mixtures more effective than
single strains? Eur J Nutr 50, 1–17 (2011).
58. Kobyliak, N., Virchenko, O. & Falalyeyeva, T. Pathophysiological role of host microbiota in the development of
obesity. Nutr J 15, 43 (2016).
59. Zhang, Q., Wu, Y. & Fei, X. Effect of probiotics on body weight and body-mass index: a systematic review and
meta-analysis of randomized, controlled trials. International Journal of Food Sciences and Nutrition 67, 571–580 (2016).
60. Koutnikova, H. et al. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related
variables: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 9, e017995 (2019).
61. Khalesi Saman, Sun Jing, Buys Nicholas & Jayasinghe Rohan. Effect of Probiotics on Blood Pressure. Hypertension
64, 897–903 (2014).
62. Tiderencel, K. A., Hutcheon, D. A. & Ziegler, J. Probiotics for the treatment of type 2 diabetes: A review of
randomized controlled trials. Diabetes/Metabolism Research and Reviews 36, e3213 (2020).
63. Leite, G. S. F., Resende Master Student, A. S., West, N. P. & Lancha, A. H. Probiotics and sports: A new magic
bullet? Nutrition 60, 152–160 (2019).
64. Shing, C. M. et al. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise
performance in the heat. Eur J Appl Physiol 114, 93–103 (2014).
65. Roberts, J. D. et al. An Exploratory Investigation of Endotoxin Levels in Novice Long Distance Triathletes, and the
Effects of a Multi-Strain Probiotic/Prebiotic, Antioxidant Intervention. Nutrients 8, 733 (2016).
66. Strasser, B. et al. Probiotic Supplements Beneficially Affect Tryptophan–Kynurenine Metabolism and Reduce the
Incidence of Upper Respiratory Tract Infections in Trained Athletes: A Randomized, Double-Blinded, Placebo-Controlled
Trial. Nutrients 8, 752 (2016).
67. Sherwin, E., Sandhu, K. V., Dinan, T. G. & Cryan, J. F. May the Force Be With You: The Light and Dark Sides of
the Microbiota-Gut-Brain Axis in Neuropsychiatry. CNS Drugs 30, 1019–1041 (2016).
68. Aizawa, E. et al. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with
major depressive disorder. Journal of Affective Disorders 202, 254–257 (2016).
69. Akkasheh, G. et al. Clinical and metabolic response to probiotic administration in patients with major depressive
disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 32, 315–320 (2016).
70. Bambling, M., Edwards, S. C., Hall, S. & Vitetta, L. A combination of probiotics and magnesium orotate attenuate
depression in a small SSRI resistant cohort: an intestinal anti-inflammatory response is suggested. Inflammopharmacol 25,
271–274 (2017).
71. Tran, N., Zhebrak, M., Yacoub, C., Pelletier, J. & Hawley, D. The gut-brain relationship: Investigating the effect of
multispecies probiotics on anxiety in a randomized placebo-controlled trial of healthy young adults. Journal of Affective
Disorders 252, 271–277 (2019).
72. Aponte, M., Murru, N. & Shoukat, M. Therapeutic, Prophylactic, and Functional Use of Probiotics: A Current
Perspective. Front. Microbiol. 11, (2020).
73. Ventura, M., Turroni, F. & van Sinderen, D. Chapter 4 - Bifidobacteria of the Human Gut: Our Special Friends. in
Diet-Microbe Interactions in the Gut (eds. Tuohy, K. & Del Rio, D.) 41–51 (Academic Press, 2015). doi:10.1016/B978-0-12-
407825-3.00004-6.
74. Ghoddusi, H. B. & Tamime, A. Y. MICROFLORA OF THE INTESTINE | Biology of Bifidobacteria. in
Encyclopedia of Food Microbiology (Second Edition) (eds. Batt, C. A. & Tortorello, M. L.) 639–645 (Academic Press, 2014).
doi:10.1016/B978-0-12-384730-0.00208-1.
75. Scardovi: Genus bifidobacterium - Google Scholar.
76. Lee, J.-H. & O’Sullivan, D. J. Genomic insights into bifidobacteria. Microbiol Mol Biol Rev 74, 378–416 (2010).
77. You, H. J., Oh, D.-K. & Ji, G. E. Anticancerogenic effect of a novel chiroinositol-containing polysaccharide from
Bifidobacterium bifidum BGN4. FEMS Microbiol Lett 240, 131–136 (2004).
78. Kim, J. Y., Park, M. S. & Ji, G. E. Probiotic modulation of dendritic cells co-cultured with intestinal epithelial cells. World J Gastroenterol 18, 1308–1318 (2012).
79. Park, J. H. et al. Encapsulated Bifidobacterium bifidum potentiates intestinal IgA production. Cell Immunol 219, 22–27 (2002).
80. Primec, M. et al. Clinical intervention using Bifidobacterium strains in celiac disease children reveals novel microbial
modulators of TNF-α and short-chain fatty acids. Clin Nutr 38, 1373–1381 (2019).
81. Quagliariello, A. et al. Effect of Bifidobacterium breve on the Intestinal Microbiota of Coeliac Children on a Gluten
Free Diet: A Pilot Study. Nutrients 8, (2016).
82. Guglielmetti, S., Mora, D., Gschwender, M. & Popp, K. Randomised clinical trial: Bifidobacterium bifidum
MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life--a double-blind, placebo-controlled
study. Aliment Pharmacol Ther 33, 1123–1132 (2011).
83. Kim, C.-S. et al. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in
community-dwelling elderly: A randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol A Biol Sci Med Sci
(2020) doi:10.1093/gerona/glaa090.
84. Milani, C. et al. Bifidobacteria exhibit social behavior through carbohydrate resource sharing in the gut. Scientific
Reports 5, 15782 (2015).
85. Milani, C. et al. Genomics of the Genus Bifidobacterium Reveals Species-Specific Adaptation to the Glycan-Rich
Gut Environment. Appl. Environ. Microbiol. 82, 980–991 (2016).
86. Duranti, S. et al. Evaluation of genetic diversity among strains of the human gut commensal Bifidobacterium
adolescentis. Scientific Reports 6, 23971 (2016).
87. LeBlanc, J. G. et al. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Current Opinion in
Biotechnology 24, 160–168 (2013).
88. Strozzi, G. P. & Mogna, L. Quantification of Folic Acid in Human Feces After Administration of Bifidobacterium
Probiotic Strains. Journal of Clinical Gastroenterology 42, S179 (2008).
89. Lucock, M. Folic Acid: Nutritional Biochemistry, Molecular Biology, and Role in Disease Processes. Molecular
Genetics and Metabolism 71, 121–138 (2000).
90. Klemenak, M., Dolinšek, J., Langerholc, T., Di Gioia, D. & Mičetić-Turk, D. Administration of Bifidobacterium
breve Decreases the Production of TNF-α in Children with Celiac Disease. Dig Dis Sci 60, 3386–3392 (2015).
91. Smecuol, E. et al. Exploratory, Randomized, Double-blind, Placebo-controlled Study on the Effects of
Bifidobacterium infantis Natren Life Start Strain Super Strain in Active Celiac Disease. Journal of Clinical Gastroenterology
47, 139–147 (2013).
92. de Almeida, N. E. C. et al. Digestion of Intact Gluten Proteins by Bifidobacterium Species: Reduction of Cytotoxicity
and Proinflammatory Responses. J. Agric. Food Chem. 68, 4485–4492 (2020).
93. O’Hara, A. M. et al. Functional modulation of human intestinal epithelial cell responses by Bifidobacterium infantis
and Lactobacillus salivarius. Immunology 118, 202–215 (2006).
94. Sibartie, S. et al. Modulation of pathogen-induced CCL20 secretion from HT-29 human intestinal epithelial cells by
commensal bacteria. BMC Immunol 10, 54 (2009).
95. Konieczna, P., Akdis, C. A., Quigley, E. M. M., Shanahan, F. & O’Mahony, L. Portrait of an immunoregulatory
bifidobacterium. Gut Microbes 3, 261–266 (2012).
96. Fu, L., Song, J., Wang, C., Fu, S. & Wang, Y. Bifidobacterium infantis Potentially Alleviates Shrimp Tropomyosin-
Induced Allergy by Tolerogenic Dendritic Cell-Dependent Induction of Regulatory T Cells and Alterations in Gut Microbiota.
Front Immunol 8, 1536 (2017).
97. Yuan, F. et al. Efficacy of Bifidobacterium infantis 35624 in patients with irritable bowel syndrome: a meta-analysis.
Curr Med Res Opin 33, 1191–1197 (2017).
98. Duar, R. M., Kyle, D. & Casaburi, G. Colonization Resistance in the Infant Gut: The Role of B. infantis in Reducing
pH and Preventing Pathogen Growth. High Throughput 9, (2020).
99. Escribano, J. et al. Bifidobacterium longum subsp infantis CECT7210-supplemented formula reduces diarrhea in
healthy infants: a randomized controlled trial. Pediatr Res 83, 1120–1128 (2018).
100. Chichlowski, M., Shah, N., Wampler, J. L., Wu, S. S. & Vanderhoof, J. A. Bifidobacterium longum Subspecies
infantis (B. infantis) in Pediatric Nutrition: Current State of Knowledge. Nutrients 12, 1581 (2020).
101. Odamaki, T. et al. Genomic diversity and distribution of Bifidobacterium longum subsp. longum across the human
lifespan. Scientific Reports 8, (2018).
102. Schell, M. A. et al. The genome sequence of Bifidobacterium longum reflects its adaptation to the human
gastrointestinal tract. PNAS 99, 14422–14427 (2002).
103. Milani, C. et al. Genomics of the Genus Bifidobacterium Reveals Species-Specific Adaptation to the Glycan-Rich
Gut Environment. Appl. Environ. Microbiol. 82, 980–991 (2016).
104. Tanaka, H., Hashiba, H., Kok, J. & Mierau, I. Bile Salt Hydrolase of Bifidobacterium longum—Biochemical and
Genetic Characterization. Appl. Environ. Microbiol. 66, 2502–2512 (2000).
105. Zhou, C. et al. Bifidobacterium longum alleviates irritable bowel syndrome-related visceral hypersensitivity and
microbiota dysbiosis via Paneth cell regulation. Gut Microbes 12, (2020).
106. Lewis, E. D. et al. Efficacy of Lactobacillus paracasei HA-196 and Bifidobacterium longum R0175 in Alleviating
Symptoms of Irritable Bowel Syndrome (IBS): A Randomized, Placebo-Controlled Study. Nutrients 12, (2020).
107. McCarville, J. L. et al. A Commensal Bifidobacterium longum Strain Prevents Gluten-Related Immunopathology in
Mice through Expression of a Serine Protease Inhibitor. Applied and Environmental Microbiology 83, (2017).
108. Lin, Y. et al. Protective role of nano-selenium-enriched Bifidobacterium longum in delaying the onset of
streptozotocin-induced diabetes. Royal Society Open Science 5, (2018).
109. Walter, J. Ecological Role of Lactobacilli in the Gastrointestinal Tract: Implications for Fundamental and Biomedical
Research. Appl. Environ. Microbiol. 74, 4985–4996 (2008).
110. Duar, R. M. et al. Lifestyles in transition: evolution and natural history of the genus Lactobacillus. FEMS Microbiol
Rev 41, S27–S48 (2017).
111. Tamang, J. P., Shin, D.-H., Jung, S.-J. & Chae, S.-W. Functional Properties of Microorganisms in Fermented Foods.
Front. Microbiol. 7, (2016).
112. Meisel, H. & FitzGerald, R. Biofunctional peptides from milk proteins. Mineral binding and cytomoduatory effects.
Current pharmaceutical design 9, 1289–95 (2003).
113. Qian, B. et al. Antioxidant, antihypertensive, and immunomodulatory activities of peptide fractions from fermented
skim milk with Lactobacillus delbrueckii ssp. bulgaricus LB340. J Dairy Res 78, 72–79 (2011).
114. Phelan, M., Aherne, A., FitzGerald, R. J. & O’Brien, N. M. Casein-derived bioactive peptides: Biological effects,
industrial uses, safety aspects and regulatory status. International Dairy Journal 19, 643–654 (2009).
115. Papadimitriou, C. G. et al. Identification of peptides in traditional and probiotic sheep milk yoghurt with angiotensin
I-converting enzyme (ACE)-inhibitory activity. Food Chemistry 105, 647–656 (2007).
116. Coda, R., Rizzello, C. G., Pinto, D. & Gobbetti, M. Selected Lactic Acid Bacteria Synthesize Antioxidant Peptides
during Sourdough Fermentation of Cereal Flours. Appl Environ Microbiol 78, 1087–1096 (2012).
117. Farvin, S., Baron, C., Nielsen, N., Otte, J. & Jacobsen, C. Antioxidant activity of yoghurt peptides: Part 2 –
Characterisation of peptide fractions. Food Chemistry 123, 1090–1097 (2010).
118. Liu, M.-M., Li, S.-T., Shu, Y. & Zhan, H.-Q. Probiotics for prevention of radiation-induced diarrhea: A meta-analysis
of randomized controlled trials. PLOS ONE 12, e0178870 (2017).
119. Rahbar Saadat, Y., Yari Khosroushahi, A. & Pourghassem Gargari, B. A comprehensive review of anticancer,
immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydrate Polymers 217,
79–89 (2019).
120. Gijsbers, L. et al. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational
studies. Am J Clin Nutr 103, 1111–1124 (2016).
121. Kommineni, S., Kristich, C. J. & Salzman, N. H. Harnessing bacteriocin biology as targeted therapy in the GI tract.
Gut Microbes 7, 512–517 (2016).
122. Hamon, E. et al. Investigation of Biomarkers of Bile Tolerance in Lactobacillus casei Using Comparative Proteomics.
J. Proteome Res. 11, 109–118 (2012).
123. van de Guchte, M. et al. Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 82, 187–216 (2002).
124. Beaufils, S. et al. The Cold Shock Response of Lactobacillus casei: Relation between HPr Phosphorylation and
Resistance to Freeze/Thaw Cycles. MIP 13, 65–75 (2007).
125. Palomino, M. M., Allievi, M. C., Gründling, A., Sanchez-Rivas, C. & Ruzal, S. M. Osmotic stress adaptation in
Lactobacillus casei BL23 leads to structural changes in the cell wall polymer lipoteichoic acid. Microbiology, 159, 2416–2426
(2013).
126. Bermudez-Brito, M., Plaza-Díaz, J., Muñoz-Quezada, S., Gómez-Llorente, C. & Gil, A. Probiotic mechanisms of
action. Ann Nutr Metab 61, 160–174 (2012).
127. Hill, D. et al. The Lactobacillus casei Group: History and Health Related Applications. Front Microbiol 9, (2018).
128. Kalliomäki, M. et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet
357, 1076–1079 (2001).
129. Johansson, M. A., Sjögren, Y. M., Persson, J.-O., Nilsson, C. & Sverremark-Ekström, E. Early colonization with a
group of Lactobacilli decreases the risk for allergy at five years of age despite allergic heredity. PLoS One 6, e23031 (2011).
130. Sanchez, M. et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and
maintenance in obese men and women. British Journal of Nutrition 111, 1507–1519 (2014).
131. Luoto, R., Kalliomäki, M., Laitinen, K. & Isolauri, E. The impact of perinatal probiotic intervention on the
development of overweight and obesity: follow-up study from birth to 10 years. International Journal of Obesity 34, 1531–
1537 (2010).
132. Novotny Núñez, I., Maldonado Galdeano, C., de Moreno de LeBlanc, A. & Perdigón, G. Evaluation of immune
response, microbiota, and blood markers after probiotic bacteria administration in obese mice induced by a high-fat diet.
Nutrition 30, 1423–1432 (2014).
133. Karimi, G. et al. The anti-obesity effects of Lactobacillus casei strain Shirota versus Orlistat on high fat
diet-induced obese rats. Food & Nutrition Research 59, (2015).
134. Tiptiri-Kourpeti, A. et al. Lactobacillus casei Exerts Anti-Proliferative Effects Accompanied by Apoptotic Cell Death
and Up-Regulation of TRAIL in Colon Carcinoma Cells. PLoS One 11, e0147960 (2016).
135. Ishikawa, H. et al. Randomized trial of dietary fiber and Lactobacillus casei administration for prevention of
colorectal tumors. Int J Cancer 116, 762–767 (2005).
136. Escamilla, J., Lane, M. A. & Maitin, V. Cell-Free Supernatants from Probiotic Lactobacillus casei and Lactobacillus
rhamnosus GG Decrease Colon Cancer Cell Invasion In Vitro. Nutrition and Cancer 64, 871–878 (2012).
137. Österlund, P. et al. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a
randomised study. British Journal of Cancer 97, 1028–1034 (2007).
138. Liu, M.-M., Li, S.-T., Shu, Y. & Zhan, H.-Q. Probiotics for prevention of radiation-induced diarrhea: A meta-analysis
of randomized controlled trials. PLOS ONE 12, e0178870 (2017).
139. Hawrelak, J. A., Whitten, D. L. & Myers, S. P. Is Lactobacillus rhamnosus GG effective in preventing the onset of
antibiotic-associated diarrhoea: a systematic review. Digestion 72, 51–56 (2005).
140. Seddik, H. A. et al. Lactobacillus plantarum and Its Probiotic and Food Potentialities. Probiotics & Antimicro. Prot.
9, 111–122 (2017).
141. Fiocco, D. et al. Characterization of the CtsR Stress Response Regulon in Lactobacillus plantarum. Journal of
Bacteriology 192, 896–900 (2010).
142. Todorov, S. D. & Franco, B. D. G. D. M. Lactobacillus Plantarum: Characterization of the Species and Application
in Food Production. Food Reviews International 26, 205–229 (2010).
143. Reid, G., Howard, J. & Gan, B. S. Can bacterial interference prevent infection? Trends Microbiol 9, 424–428 (2001).
144. Zheng, Y. et al. Probiotic properties of Lactobacillus strains isolated from Tibetan kefir grains. PLoS One 8, e69868
(2013).
145. Liu, Y.-W., Liong, M.-T. & Tsai, Y.-C. New perspectives of Lactobacillus plantarum as a probiotic: The gut-heart-
brain axis. J Microbiol. 56, 601–613 (2018).
146. Rudzki, L. et al. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive
functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology
100, 213–222 (2019).
147. Rudzki, L. & Szulc, A. ‘Immune Gate’ of Psychopathology-The Role of Gut Derived Immune Activation in Major
Psychiatric Disorders. Front Psychiatry 9, 205 (2018).
148. Liu, Z. et al. Lactobacillus plantarum prevents the development of colitis in IL-10-deficient mouse by reducing the
intestinal permeability. Mol Biol Rep 38, 1353–1361 (2011).
149. Yin, M. et al. Micro Integral Membrane Protein (MIMP), a Newly Discovered Anti-Inflammatory Protein of
Lactobacillus Plantarum, Enhances the Gut Barrier and Modulates Microbiota and Inflammatory Cytokines. Cell Physiol
Biochem 45, 474–490 (2018).
150. Qin, H.-L. et al. Effect of Lactobacillus plantarum enteral feeding on the gut permeability and septic complications
in the patients with acute pancreatitis. Eur J Clin Nutr 62, 923–930 (2008).
151. Vanderpool, C., Yan, F. & Polk, D. B. Mechanisms of probiotic action: Implications for therapeutic applications in
inflammatory bowel diseases. Inflamm Bowel Dis 14, 1585–1596 (2008).
152. Jang, S.-E., Han, M. J., Kim, S.-Y. & Kim, D.-H. Lactobacillus plantarum CLP-0611 ameliorates colitis in mice by
polarizing M1 to M2-like macrophages. Int Immunopharmacol 21, 186–192 (2014).
153. Chu, Z.-X. et al. Lactobacillus plantarum prevents the upregulation of adhesion molecule expression in an
experimental colitis model. Dig Dis Sci 55, 2505–2513 (2010).
154. Mitall, B. K. & Garg, S. K. Anticarcinogenic, Hypocholesterolemic, and Antagonistic Activities of Lactobacillus
acidophilus. Critical Reviews in Microbiology 21, 175–214 (1995).
155. Pfeiler, E. A. & Klaenhammer, T. R. Role of transporter proteins in bile tolerance of Lactobacillus acidophilus. Appl
Environ Microbiol 75, 6013–6016 (2009).
156. Ringel-Kulka, T. et al. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus
placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol
45, 518–525 (2011).
157. Leyer, G. J., Li, S., Mubasher, M. E., Reifer, C. & Ouwehand, A. C. Probiotic effects on cold and influenza-like
symptom incidence and duration in children. Pediatrics 124, e172-179 (2009).
158. Homayouni, A. et al. Effects of probiotics on the recurrence of bacterial vaginosis: a review. J Low Genit Tract Dis
18, 79–86 (2014).
159. Auclair, J., Frappier, M. & Millette, M. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and
Lactobacillus rhamnosus CLR2 (Bio-K+): Characterization, Manufacture, Mechanisms of Action, and Quality Control of a
Specific Probiotic Combination for Primary Prevention of Clostridium difficile Infection. Clin Infect Dis 60 Suppl 2, S135-
143 (2015).
160. Macouzet, M., Lee, B. H. & Robert, N. Production of conjugated linoleic acid by probiotic Lactobacillus acidophilus
La-5. J Appl Microbiol 106, 1886–1891 (2009).
161. Cook, D. P., Gysemans, C. & Mathieu, C. Lactococcus lactis As a Versatile Vehicle for Tolerogenic Immunotherapy.
Front Immunol 8, 1961 (2017).
162. Cook, D. P., Gysemans, C. & Mathieu, C. Lactococcus lactis As a Versatile Vehicle for Tolerogenic Immunotherapy.
Front Immunol 8, 1961 (2017).
163. Luerce, T. D. et al. Anti-inflammatory effects of Lactococcus lactis NCDO 2118 during the remission period of
chemically induced colitis. Gut Pathog 6, 33 (2014).
164. Martín, R. et al. Effects in the use of a genetically engineered strain of Lactococcus lactis delivering in situ IL-10 as
a therapy to treat low-grade colon inflammation. Hum Vaccin Immunother 10, 1611–1621 (2014).






